The features of SONDELBAY® and its pen were selected with patients in mind1,2
SONDELBAY® offers people with osteoporosis a small and light-weight device for self-injecting teriparatide, designed to be easy to handle:²
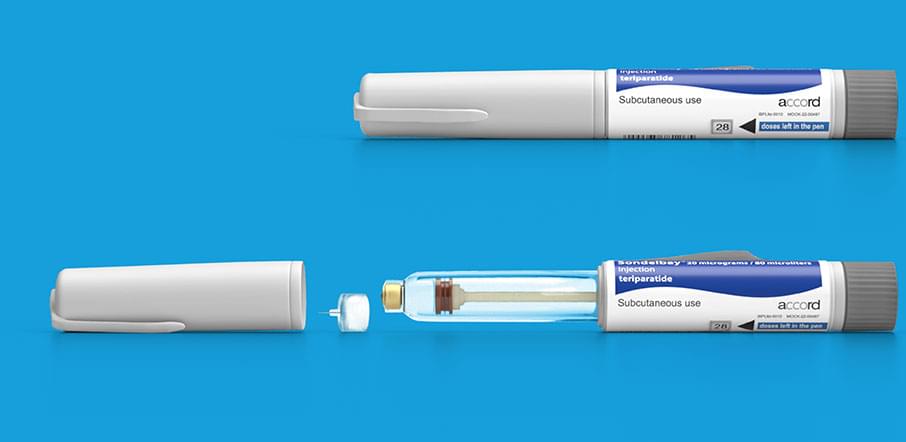
- Compact and light-weight pen²
- Side injection button³
- Visible dose counter³
- No priming needed³
- No removing and replacing of cartridges³
- Clear view of the drug solution in the pen once the pen cap is removed³
- Maximum 3 days out-of-fridge stability at temperatures up to 25°C¹
- In-use stability demonstrated for 28 days at 2-8°C. Within the 28 days, the product can be stored at temperature conditions up to 25°C for a maximum of 3 days. Once opened, it may be stored for a maximum of 28 days at the above temperature conditions¹
References:
- SONDELBAY® Summary of Product Characteristics.
- Data on file – UK-03873 (March 2022). Sondelbay Product Details.
- SONDELBAY® Instructions for use.